PRÊT1D – National project for the screening and management of presymptomatic type 1 diabetes, NEWLY SUPPORTED by the FFRD
PRÊT1D – National project for the screening and management of presymptomatic type 1 diabetes, NEWLY SUPPORTED by the FFRD
The recently published French position statement by the SFD/SFEDP provides an initial reference framework for the screening and management of preclinical type 1 diabetes (T1D) in relatives of individuals living with T1D. In addition, new ICD-10 diagnostic codes have been created for stage 1 (E10.A1) and stage 2 (E10.A2) T1D, suggesting that the management of preclinical T1D following screening is moving toward integration into routine clinical care
The PRÊT1D study, promoted by the Fondation Francophone pour la Recherche sur le Diabète (FFRD ), aims to remove 3 barriers to the development of this new patient pathway:
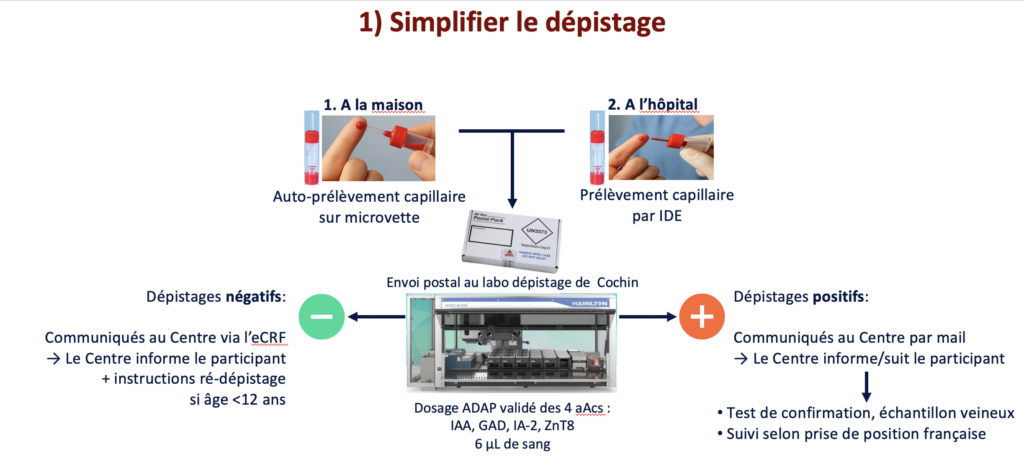
PRÊT1D proposes to simplify this step, which represents the largest volume of testing, and to move it outside hospitals and private practices by using capillary blood samples that can be self-collected at home and sent by mail to a centralized screening laboratory at Cochin Hospital. The four T1D-associated autoantibodies (IAA, GAD, IA-2, ZnT8) are measured there using a validated ADAP assay. Then, each screening result is communicated to the referring center, which informs the participant and organizes follow-up in accordance with the French position statement. An option for sample collection by healthcare professionals at participating centers is also available.
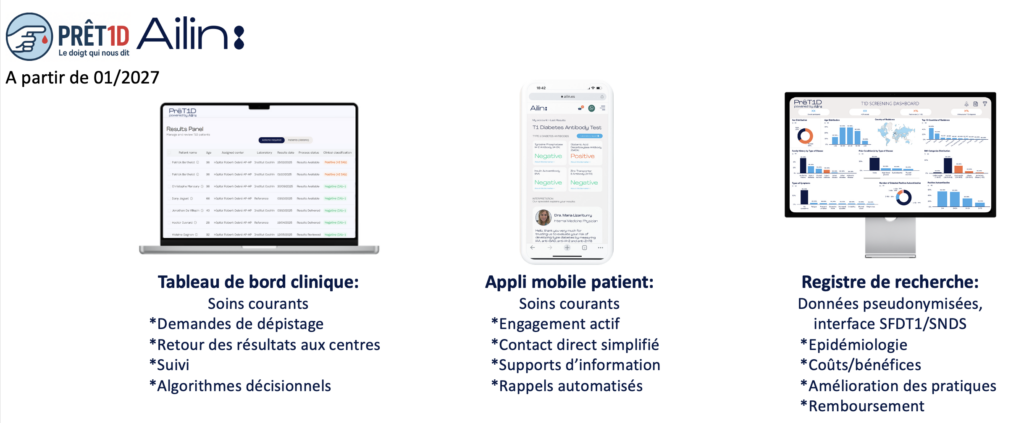
The creation of a harmonized follow-up and care pathway for relatives with a positive screening test. Bu the end, PRÊT1D is proposing to simplify the process, using a digital health platform developed with startup Ailin. This platform will include 3 modules:
- a) a clinical dashboard for professionals, centralizing screening requests, results and follow-up with an individual, family and global vision, and proposing medical decision support algorithms;
- b) a mobile application for patients, a tool for active engagement, facilitating direct patient/expert center contact and offering information support and automated reminders;
- c) a research registry, a pseudonymized database covering the prevalence/natural history of preclinical T1DM, the acceptability/feasibility of screening and the cost of screening/follow-up.
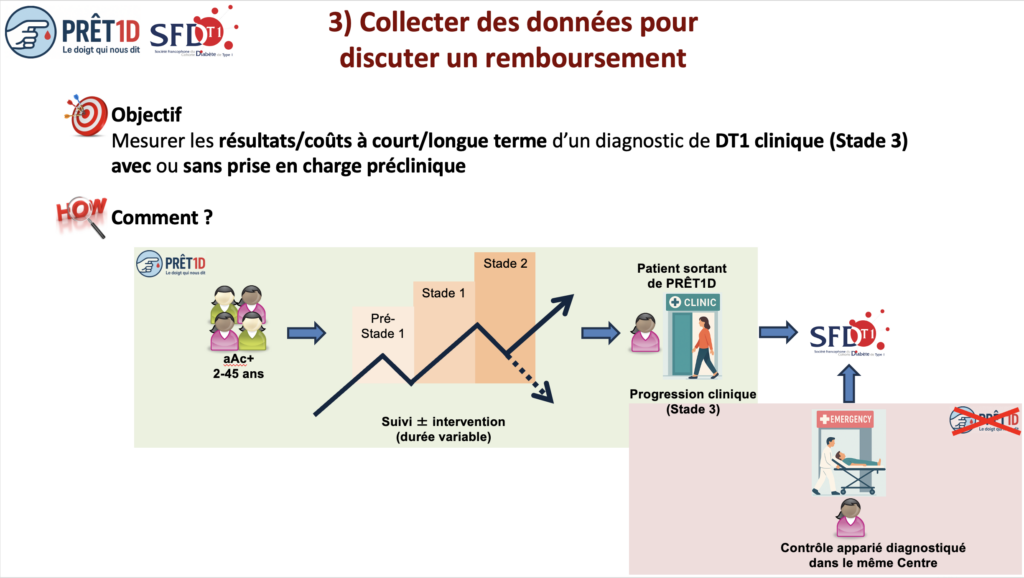
PRÊT1D proposes to collect the data needed to discuss reimbursement options with the HAS using an interface with the SFDT1 cohort, a study also promoted by the Fondation Francophone pour la Recherche sur le Diabète (FFRD) since 2019. This interface will make it possible to track individuals who have progressed to clinical T1DM and compare them with those diagnosed without screening, at the time of clinical declaration and then at a distance: glycemic control and micro/macroangiopathic complications via the SFDT1costs incurred/avoided via SNDS data not available in other countries.
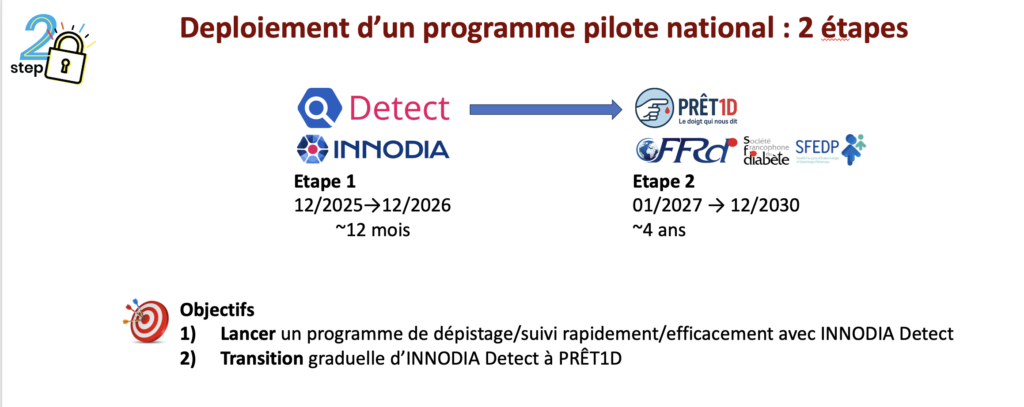
This national pilot project (29 centers by 12/25/2025) will be rolled out in 2 steps:
On a small scale: 2,900 screenings/year for around 12 months through the INNODIA Detect protocol, scheduled to start up in January 2026.
On a larger scale: 7,500 screenings/year for 4 years, through the PRÊT1Dprotocol, which will gradually follow.